Serum-free Medium for Vero Cells

ToCell® Vero CD is a serum-free, chemically defined, and animal-component-free adherent medium, specifically formulated for Vero cells.
|
● Chemically defined
|
● Serum-free
|
|
● High proliferation rate
|
● Animal-free components
|
|
● No cell acclimation required
|
|
Applicable for large-scale industrial production of vaccines, such as COVID-19, rabies (for human and veterinary use), Japanese encephalitis (JE), hepatitis A, inactivated poliovirus (IPV), EV71, rotavirus, veterinary rabies, and porcine epidemic diarrhea vaccines.
|
Product |
Product Code |
Packaging Specifications |
|
Vero SFM |
633D |
1L、10L、25L、50L、100L、200L、250L、300L |
|
Vero VM (Virus maintenance solution) |
632 |
1L、10L、25L、50L、100L、200L、250L、300L |
- Case Presentation
-
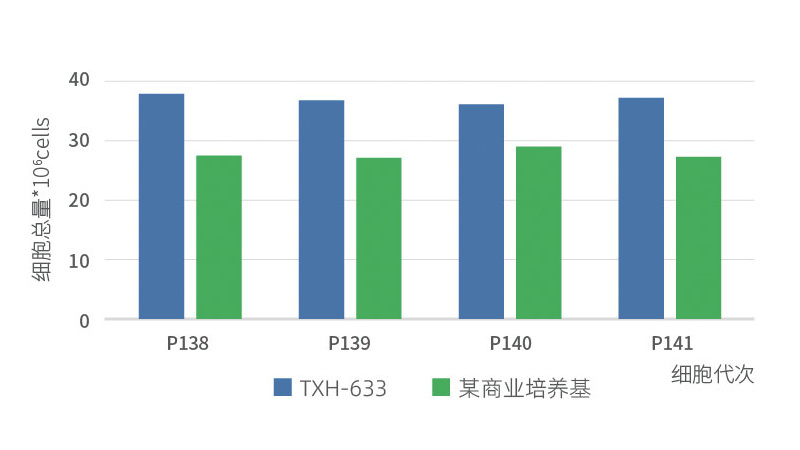
Comparative experiment with commercial culture medium
Continuous passage data of Vero cells in different serum-free cultures Cell: Vero cell
Culture medium: Vero cell serum-free medium (633)/a commercial medium
Total vaccination amount: 7×106cells
Pancreatic enzyme: TrypLETM Express Enzyme(1X)GIBCO
Cultivation conditions: 37℃、5%CO₂、T75 Square bottle (breathable cap)
Exposure dose: 1/200Virus: PV strain
Continuous production process of rabies virus without serum in 14L DISK reactor
Continuous production of rabies vaccine through serum-free culture in a 14L DISK fixed bed bioreactor
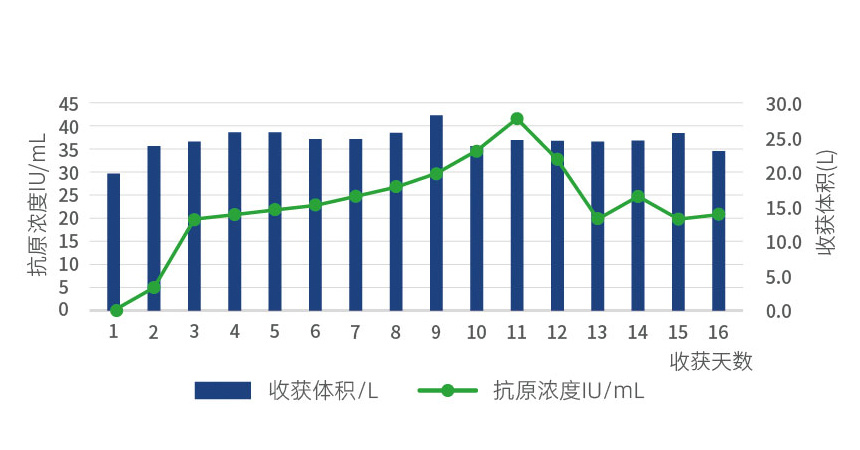
The results showed that TXH-633 serum-free medium was amplified from the cell factory and inoculated into a 14L DISK reactor for continued serum-free culture, followed by inoculation with rabies virus. During the continuous harvesting process of viral antigens, there were 14 days where the harvested antigen levels were greater than 20IU/mL, and the process could be further scaled up to meet the large-scale production needs of rabies vaccines for human or animal use.

Official account

Wuxi Address: 699 Zhide Avenue, Xinwu District, Wuxi City
Suzhou Address: Entrance A, 1F, Zhanhua Science and Technology Building, No. 8 Pangyang Road, Wujiang District, Suzhou City, Jiangsu Province
Copyright:© 2025 CNBG ToCell (Wuxi) Biotechnology Co., Ltd. Powered by:www.300.cn SEO
