New product release! ToCell's localized paper carrier - Cellcomb carrier is launched!
Release Time:
2021-08-24
The Cellcomb carrier, a domestically produced paper carrier with complete independent intellectual property rights and fully localized raw materials, key technologies, and core processes, has been launched!
 Independent and controllable full localization of paper carriers to solve the "bottleneck" problem
Independent and controllable full localization of paper carriers to solve the "bottleneck" problem
ToCell (Suzhou) Biotechnology Co., Ltd. (hereinafter referred to as: ToCell Suzhou Company) has broken through the technological blockade of foreign raw material and equipment manufacturers. Through the technological innovation of the R&D team, it has developed a domestically produced paper carrier - Cellcomb TM carrier, which is fully localized from raw materials, key technologies to core processes and has completely independent intellectual property rights.

▲ Actual photo of Cellcomb TM vector
CellcombTMIntroduction to Cellcomb TM Vectors
The Cellcomb TM carrier produced by ToCell Suzhou is suitable for the growth of anchorage-dependent cells. It uses high-purity polyester fibers suitable for cell attachment growth. The materials used meet the requirements of "USP<88> plastic classification VI", have an efficiently usable surface area and a three-dimensional spatial structure, which is more conducive to the good growth and proliferation of cells, and is also conducive to the transfer of various nutrients and oxygen, achieving high-density large-scale cell culture.
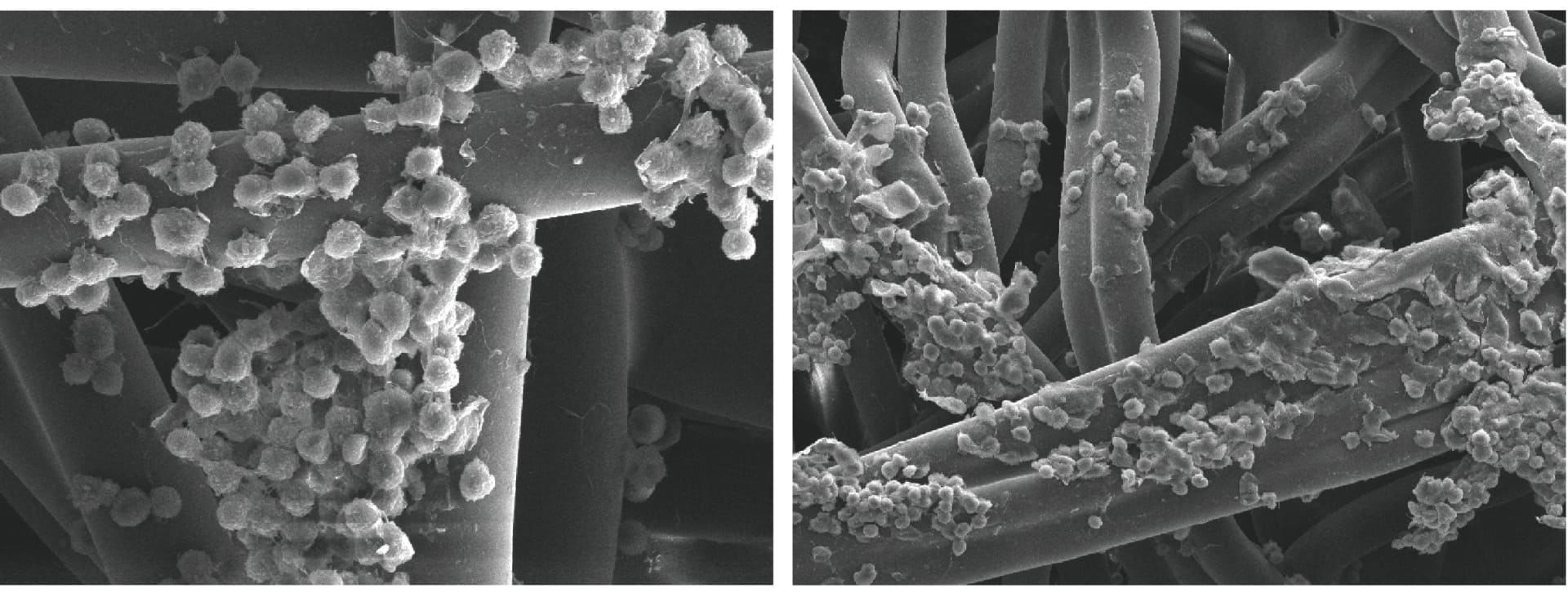
▲ Electron microscope scanning image of Cellcomb TM carrier
CellcombTM Cellcomb TM Vector Production
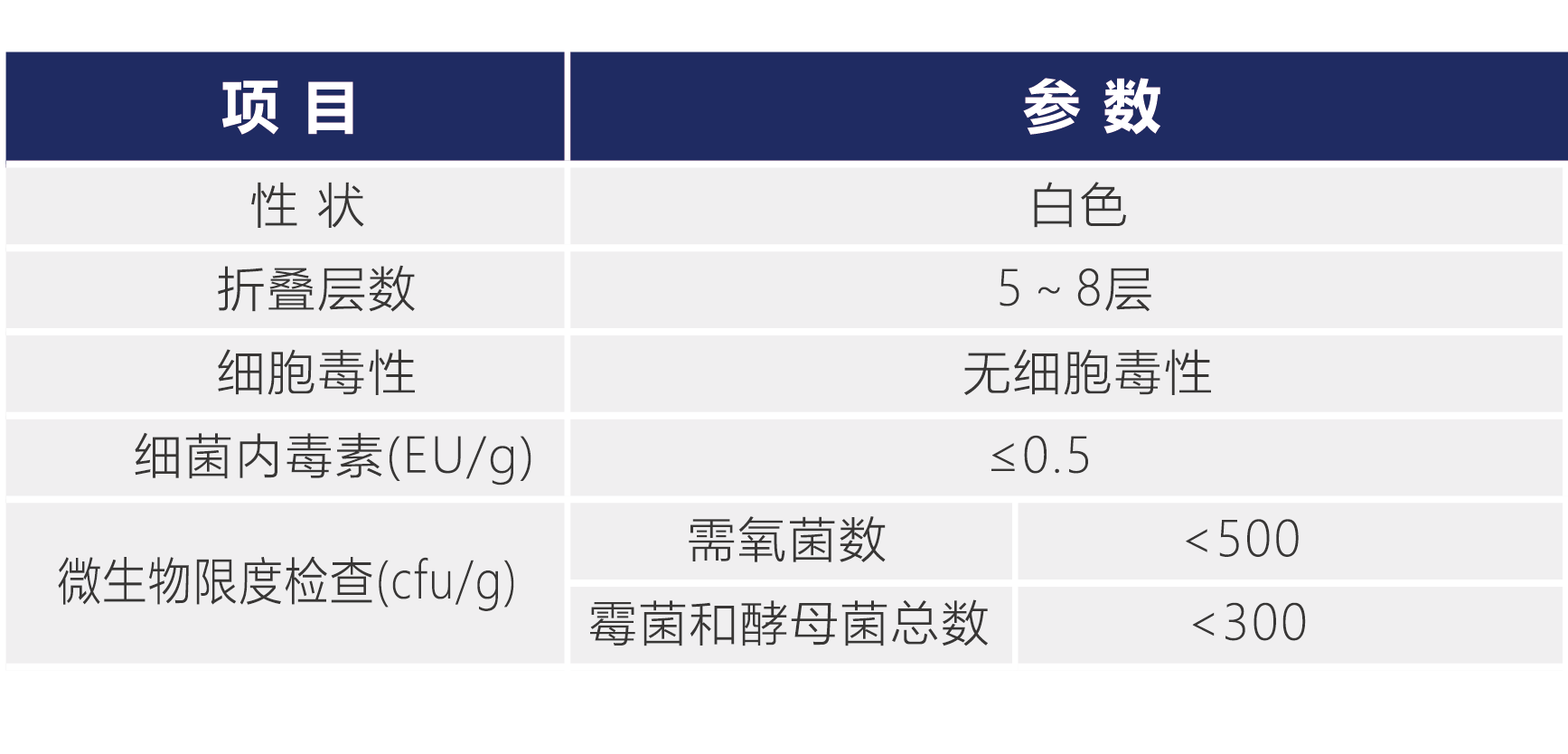
Cellcomb TM vectors are produced in a Class D clean area. The production process strictly follows GMP specifications, with strict requirements on personnel, facilities and equipment, production process, quality control, etc. to ensure product quality. There are full-time production personnel and quality management personnel, and the production process is monitored on-site by QA. Complete raw material quality standards and finished product quality standards have been established. Each batch is tested, and a test report is issued after the test is qualified. Raw material testing items include properties, dissolution, and biological testing; finished product testing items include properties, number of folding layers, cytotoxicity, bacterial endotoxins, and microbial limits.
CellcombTMCellcomb TM Vector Parameters
The establishment of a domestic carrier platform can not only shorten the process development process and significantly reduce the cost of biopharmaceutical development, but also enhance China's competitiveness in the field of biopharmaceuticals. ToCell will continue to create value for the industry and customers, ensure the stability and completeness of the biopharmaceutical industry supply chain, develop China's biopharmaceutical industry support technology, and contribute to solving the "bottleneck" project of biopharmaceuticals. ToCell will continue to create value for the industry and customers, ensure the stability and completeness of the biopharmaceutical industry supply chain, develop China's biopharmaceutical industry support technology, and contribute to solving the "bottleneck" project of biopharmaceuticals.
Bonus at the end of the article: New products are on the market, scan the QR code to make an appointment to try them first

CellcombTMCellcomb TM vector product manual, please go to [ Home > Technical Support > Download Center ] to download
More News

Official account

Wuxi Address: 699 Zhide Avenue, Xinwu District, Wuxi City
Suzhou Address: Entrance A, 1F, Zhanhua Science and Technology Building, No. 8 Pangyang Road, Wujiang District, Suzhou City, Jiangsu Province
Copyright:© 2025 CNBG ToCell (Wuxi) Biotechnology Co., Ltd. Powered by:www.300.cn SEO

